Research projects – Group A. Bernut
AXIS 1 : ASSESS HOW CFTR INFLUENCES THE ANTI-INFECTIOUS RESPONSE OF PROFESSIONAL PHAGOCYTES
Patients with cystic fibrosis (CF) are particularly vulnerable to infections by various bacterial pathogens such as Staphylococcus aureus, Pseudomonas aeruginosa or certain atypical mycobacteria such as Mycobacterium abscessus.
We recently set up zebrafish models lacking functional CFTR. These models recapitulate the susceptibility towards bacterial pathogens observed in CF patients. In particular, our work shows that this susceptibility to infections in CFTR-deficient zebrafish is partly conditioned by a less effective antibacterial activity of professional phagocytes, thus impairing efficient fight against pathogens.
Through the combined use of fluorescent pathogens and transgenic lines labelling immune cells, our goal is to define the role of CFTR in the regulation of bactericidal defenses orchestrated by professional phagocytes against different pathogens representative of the disease, and therefore to understand how an imbalance leads to uncontrolled infections in CF.
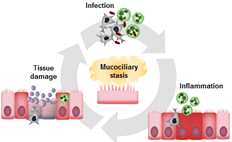
AXIS 2 : ASSESS HOW CFTR REGULATES THE INFLAMMATORY POTENTIAL OF EPITHELIAL AND MYELOID CELLS, AND HOW IT MODULATES TISSUE REPAIR
Chronic neutrophil-dominant inflammation is one of the hallmarks of the lung pathology associated with cystic fibrosis, and it eventually causes the premature death of patients. If for a long time, infection was perceived as the initiating element of the inflammation in patients’ airways, certain observations show the presence of a pre-existing inflammation before any sign of infection in the lungs.
Using zebrafish larvae, our work reveals that a loss of CFTR alters the epithelial response to non-infectious lesions and leads to excessive and persistent neutrophilic inflammation. This uncontrolled inflammation results in a lack of tissue repair.
We seek to define the role of CFTR in the regulation of the inflammatory and regenerative potential of the host, with the aim of obtaining a better characterization of the mechanisms involved in the persistence of inflammation and in the failure of tissue repair during cystic fibrosis. To do this, we will exploit the zebrafish larva model in a normal or mucoviscidal context, and we will study the activity of epithelial and myeloid cells during inflammatory and regenerative processes in the context of septic or aseptic inflammation.
AXIS 3 : PROPOSE NEW THERAPEUTIC ALTERNATIVES FOR PATIENTS WITH CYSTIC FIBROSIS
Reducing the deleterious effects of infection and inflammation is a key element in the care of CF patients. The identification of the molecular and cellular processes underlying inflammation and infection in CF provides information on the most interesting immune mechanisms to optimize or inhibit from a therapeutic perspective.
Our goal is to identify and characterize anti-inflammatory, pro-resolving and/or immuno-modulating compounds capable of rebalancing innate immunity and restoring epithelial integrity, or even controlling infection during CF.
To do this, we will use the zebrafish larva because its major advantage is to be able to visualize the therapeutic action of a molecule on inflammation or infection in vivo.
MODEL ORGANISMS:
Mycobacterium abscessus
Staphylococcus aureus
Pseudomonas aeruginosa
Zebrafish
BIOLOGICAL PROCESSES STUDIED:
Host-pathogen interactions
Inflammation
USED TECHNIQUES:
Animal experimentation
Bacteriology
Phenotype screening
MEDICAL APPLICATIONS:
Identification of potential targets for the development of immuno-modulatory molecules
Identification of molecules capable of restoring innate immunity in the context of cystic fibrosis