Research projects – Group L. Yatime
The detection of pathogen- and damage-associated molecular patterns (PAMPs and DAMPs) by conventional actors of the innate system (complement system or Toll-like receptors, for example) has been studied in depth. On the other hand, vertebrates possess many other such sensors still needing to be characterized. Our current research efforts focus on several scavenger receptors from the immunoglobulin superfamily involved in the recognition of endogenous alarmins or pathogenic virulence factors, all linked to inflammatory conditions in humans.
Receptors from the immunoglobulin superfamily (IgSF) are membrane receptors that only contain immunoglobulin (Ig) domains on their extracellular region. Since Ig domains tend to homodimerize either in cis or trans, many IgSF receptors are prone to homophilic and/or heterophilic interactions. As a result, they are often involved in cell adhesion processes and they contribute to numerous vital cellular functions including cell motility, proliferation and differentiation, reorganization of the cytoskeleton, neuronal growth or immune response. Due to their adhesive properties, these receptors also play a central role in many human pathologies, notably cancer and infections, and they are very robust vectors of inflammation.
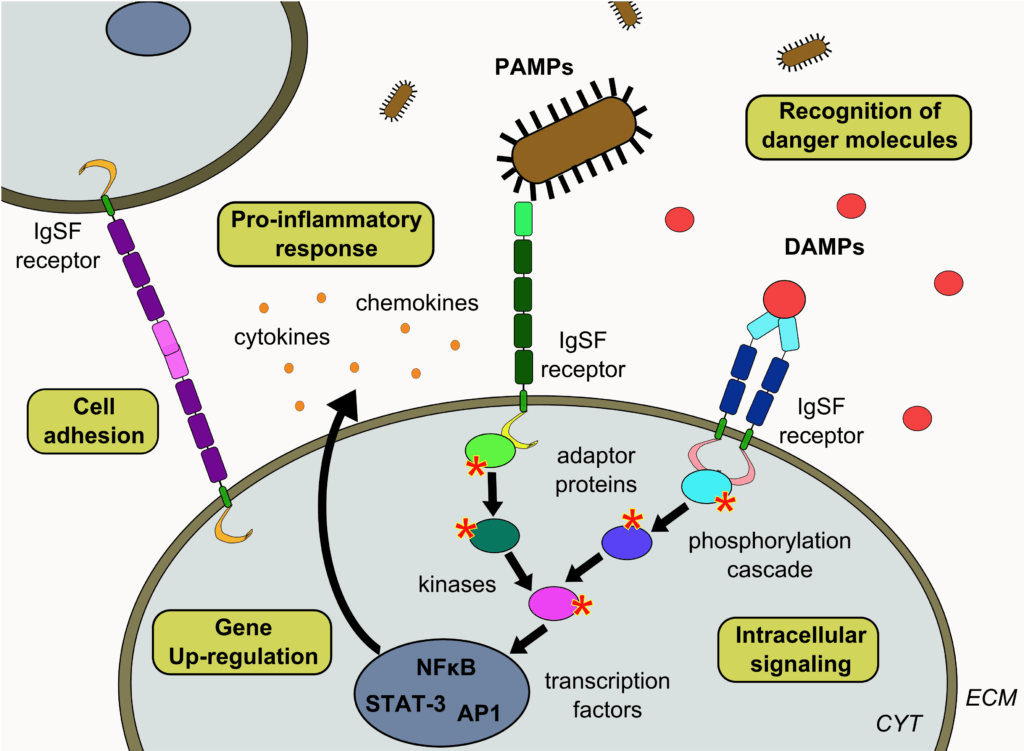
Figure 1 : IgSF receptors and their role in cell adhesion processes and in the recognition of danger molecules (DAMPs and PAMPs)
Among this large family of receptors, we are interested in two sub-classes involved in the recognition of danger stress signals that are either endogenous (DAMPs) or pathogen-associated (PAMPs):
– the multiligand receptor RAGE, which detects a wide range of endogenous alarmins implicated in cancer, diabetes or neurodegenerative disorders
– the CEACAM receptors responsibles for the recognition of various intestinal pathogens in a context of inflammatory bowel disease (IBD)
AXIS 1 : SENSING OF S100 ALARMINS BY THE RAGE RECEPTOR DURING CANCER
S100 proteins, specific for vertebrates, are normally found inside cells. Upon calcium binding, they regulate calcium-dependent, intracellular processes including transcription, cytoskeletal dynamics, cell growth and differentiation, and calcium homeostasis. In a context of cellular stress and inflammation related to the development of cancer, S100 proteins are overexpressed and can be released into the extracellular environment where they become danger molecules recognized by the RAGE receptor. This sensing induces a pro-inflammatory response which promotes tumor progression. In consequence, the RAGE-S100 interactions are considered as promising new targets for the development of novel anti-cancer therapies.
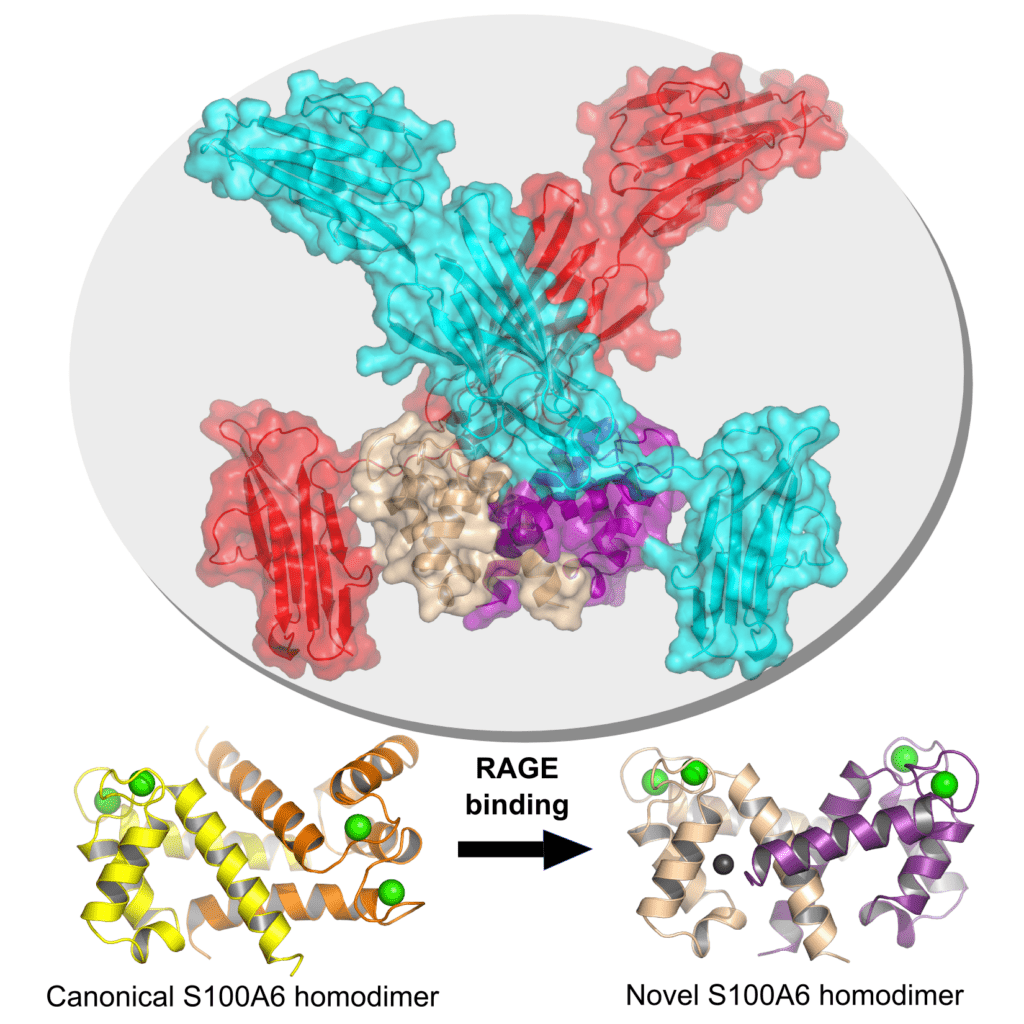
Figure 2 : Crystal structure of the RAGE-S100A6 complex at 2.3 Å resolution (upper). Apo canonical form (lower, left) and novel form observed upon binding to RAGE (lower, right) for the S100A6 homodimer.
Our work, based on biochemical and structural approaches, as well as in vivo modeling in zebrafish, aims to elucidate the mechanisms of recognition of these S100 proteins by the RAGE receptor, in order to design therapeutic inhibitors targeting these interactions. We recently managed to determine the first crystallographic structure of a full-length complex between RAGE and the S100A6 protein. This structure revealed a new homodimeric form of S100A6, potentially responsible for the pro-inflammatory activity of the protein. Our current efforts are focused on the analysis of other RAGE-S100 complexes, to determine if this new form is observed for other S100 proteins. We also investigate how divalent ions and oxidative properties met in the extracellular matrix affect the 3D architecture of these proteins and what is the associated impact on their inflammatory properties.
AXIS 2 : RECOGNITION OF INTESTINAL PATHOGENS BY THE CEACAM RECEPTORS DURING IBD
Inflammatory bowel diseases constitue a real public health problem due to their increasing incidence, notably in developed countries where diet (fat and sugar), lifestyle (stress, alcohol, smoking) and environmental conditions (pollution, low sunlight) favor their development. The gut microbiota plays an essential role in the pathogenesis of these diseases through repeated deleterious interactions with the host cells of the intestinal epithelium, in individuals weakened by genetic deficiencies. This contributes to the maintenance of a high inflammatory state which represents the major pathological manifestation in IBDs such as Crohn’s disease or ulcerative colitis.
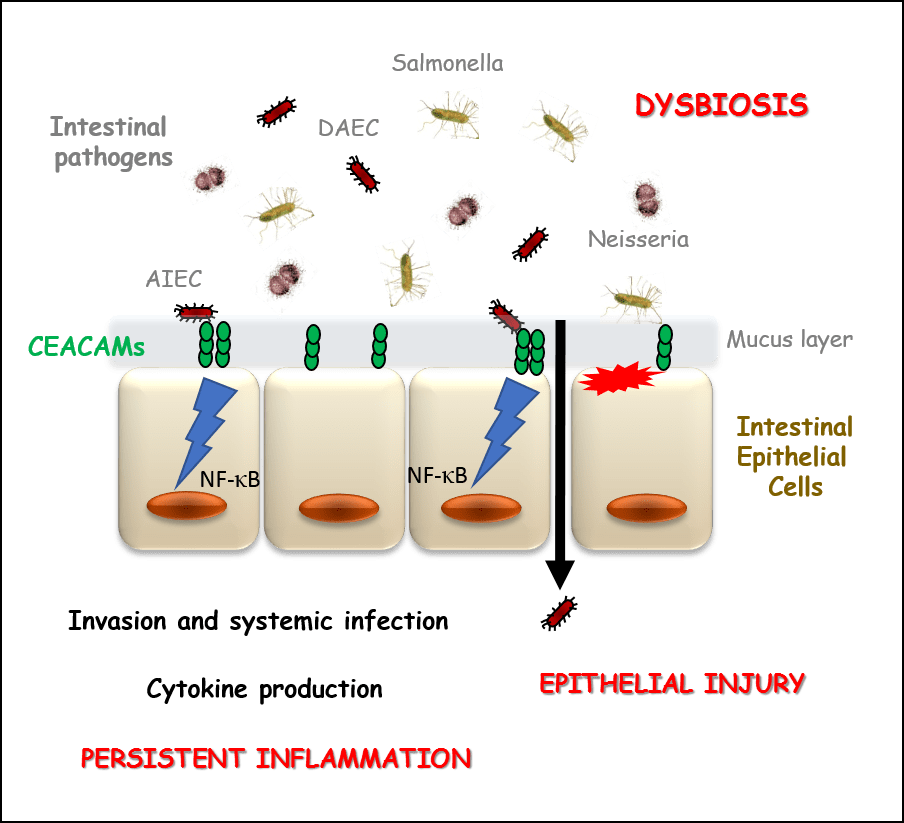
Figure 3 : The CEACAM-pathogene interactions promote the persistence of a high inflammatory state during IBDs.
CEACAMs receptors, present on the intestinal epithelium, are used as anchoring points by intestinal pathogens such as Adherent Invasive Escherichia coli (AIEC), Diffusively Adherent Escherichia coli (DAEC), neisserial strains or salmonella. These host-pathogen interactions generate an exacerbated inflammatory response, through the production of pro-inflammatory cytokines (TNF-alpha, interleukins), which can no longer be controlled by our body. This process allows the internalisation of the pathogens and their immune escape, which in turn perpetuates the high inflammatory state. We seek to characterize the precise mode of recognition of these intestinal pathogens by various members of the CEACAM family, in order to design specific inhibitors of these interactions for application in the treatment of IBDs.