Research Projects – Group S. Besteiro
Research project. New metabolic targets to combat toxoplasmosis
Like several other medically important parasites of the phylum Apicomplexa (ie malaria-causing Plasmodium), T. gondii contains an unusual organelle called the apicoplast. This organelle is a startling peculiarity that not only highlights the diversity of eukaryotic cell biology, but can also potentially be leveraged for therapeutic development. This four-membrane plastid was acquired by an unusual secondary endosymbiosis, in which an alga was engulfed by another eukaryote forming a new secondary plastid in the host. Although the apicoplast has lost its photosynthetic function, it houses several important metabolic pathways for generating: iron/sulfur cluster, hem, fatty acids (FASII), isoprenoids (Fig. 1).
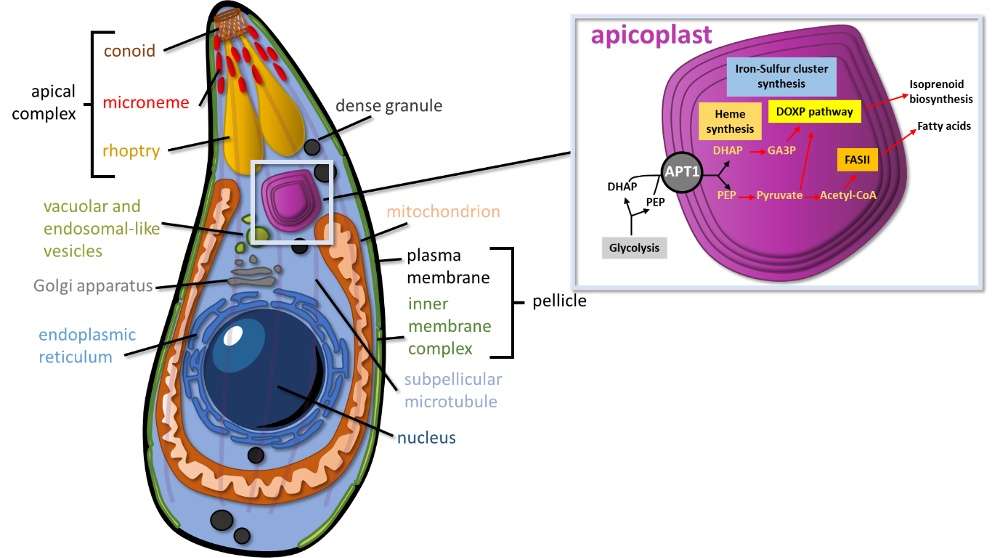
Figure 1. Schematic representation of a Toxoplasma tachyzoite, with a four-membrane apicoplast and the main biochemical pathways it contains.
Although the apicoplast is known as an important metabolic hub for many species of apicomplexan parasites, the metabolic pathways which are absolutely essential for parasite viability may vary depending on the parasite or the developmental stage. For instance, T. gondii has a complex life cycle involving several developmental stages that develop in felids (the definitive hosts, where sexual reproduction occurs), but also divide asexually in the many species of warm-blooded animals that can act as intermediate hosts. The two developmental forms found in intermediate hosts are the tachyzoite and the bradyzoite. Tachyzoites are rapidly dividing forms associated with the acute phase of toxoplasmosis. Upon control by a competent immune system, however, the parasites can differentiate into slowly-growing bradyzoites, establishing within tissue cysts and responsible for the chronic phase of toxoplasmosis.
The apicoplast is already targeted by anti-parasitic drugs (for instance through several prokaryotic translation inhibitors) and apicoplast-hosted hem, fatty acid and isoprenoid synthesis pathways have all been shown to contribute to the fitness of the tachyzoite stage. However, several metabolic pathways remain unexplored and unexploited as potential drug targets.
Although there are effective medicines available against tachyzoites, the persistent chronic form of the pathogen remains in the host throughout its life and can convert repeatedly back into tachyzoites, and hence lead to a severe pathology (i.e. encephalitis or retinitis) in the event of a weakened immune system. These bradyzoites forms are thus central to the pathology, yet there are no effective drugs against them so far.
In bradyzoites, where apicoplast function has been largely overlooked, we have recently demonstrated the importance of the apicoplast for survival and persistence of this parasite stage. It validates this organelle as a potential drug target for combating toxoplasmosis.
Over the last few years, we have focused on the iron-sulfur cluster synthesis pathways, one of which (the SUF pathway) is hosted by the apicoplast and is essential for tachyzoite viability, while being absent from the mammalian hosts of the parasite. Yet, even in pathways generally conserved in eukaryotes like the cytosolic iron-sulfur cluster assembly pathway (CIA pathway), some lineage-specific features exist in Apicomplexa. We have for instance recently uncovered an unusual and essential function of protein HCF101 in the CIA pathway of T. gondii, while this Fe-S cluster transfer protein is absent from mammals.
Latest publications related to this project:
Renaud EA, Maupin AJM, Berry L, Bals J, Bordat Y, Demolombe V, Rofidal V, Vignols F, Besteiro S. The HCF101 protein is an important component of the cytosolic iron-sulfur synthesis pathway in Toxoplasma gondii. PLoS Biol. 23(2):e3003028. doi: 10.1371/journal.pbio.3003028
Renaud EA, Pamukcu S, Cerutti A, Berry L, Lemaire-Vieille C, Yamaryo-Botté Y, Botté CY, Besteiro S (2022) Disrupting the plastidic iron-sulfur cluster biogenesis pathway in Toxoplasma gondii has pleiotropic effects irreversibly impacting parasite viability. J Biol Chem 298:102243. doi:10.1016/j.jbc.2022.102243
Sanchez SG, Bassot E, Cerutti A, Mai Nguyen H, Aïda A, Blanchard N, Besteiro S (2023) The apicoplast is important for the viability and persistence of Toxoplasma gondii bradyzoites. Proc Natl Acad Sci USA 120:e2309043120. doi:10.1073/pnas.2309043120
Pamukcu S, Cerutti A, Bordat Y, Hem S, Rofidal V, Besteiro S (2021) Differential contribution of two organelles of endosymbiotic origin to iron-sulfur cluster synthesis and overall fitness in Toxoplasma. PLoS Pathog 17:e1010096. doi:10.1371/journal.ppat.1010096
Other research projects
We are also interested in other aspects of the cell biology of Toxoplasma, including specific aspects of the cell division process, the cytoskeleton, as well as canonical and non-canonical functions of the autophagy machinery.
This includes:
► elucidating the physiological roles of parasite autophagy in the context of acute and chronic toxoplasmosis (collaboration with the labs of Ellen Yeh, Stanford Uni., USA and of Vern Carruthers, Univ. Michigan, USA)
► identifying novel parasite-specific functions for the autophagy-related machinery at the apicoplast
Latest publications related to this project:
Walczak M, Meister TR, Nguyen HM, Zhu Y, Besteiro S, Yeh E. (2023) Structure-Function Relationship for a Divergent Atg8 Protein Required for a Nonautophagic Function in Apicomplexan Parasites. mBio. Feb 28;14(1):e0364221. doi: 10.1128/mbio.03642-21.
Smith D, Kannan G, Coppens I, Wang F, Nguyen HM, Cerutti A, Olafsson EB, Rimple PA, Schultz TL, Mercado Soto NM, Di Cristina M, Besteiro S, Carruthers VB. (2021) Toxoplasma TgATG9 is critical for autophagy and long-term persistence in tissue cysts. Elife. Apr 27;10:e59384. doi: 10.7554/eLife.59384.
Finally, we are using T. gondii as a model to elucidate the mode of action of antiparasitic drugs targeting redox or iron metabolism.
Latest publications related to these projects:
Renaud EA, Maupin AJM, Bordat Y, Graindorge A, Berry L, Besteiro S. (2024)
Iron depletion has different consequences on the growth and survival of Toxoplasma gondii strains.Virulence (1):2329566.doi: 10.1080/21505594.2024.2329566
Dupouy B, Donzel M, Roignant M, Charital S, Keumoe R, Yamaryo-Botté Y, Feckler A, Bundschuh M, Bordat Y, Rottmann M, Mäser P, Botté CY, Blandin SA, Besteiro S, Davioud-Charvet E. (2024) 3-Benzylmenadiones and their Heteroaromatic Analogues Target the Apicoplast of Apicomplexa Parasites: Synthesis and Bioimaging Studies. ACS Infect Dis. 11;10(10):3553-3576. doi: 10.1021/acsinfecdis.4c00304