Egress and protein phosphatases in P. falciparum
Head of group: Dr. Mauld Lamarque
EGRESS AND THE PARASITE PHOSPHATASE PP1 IN PLASMODIUM FALCIPARUM
Project on egress and the parasite phosphatase PP1
Asexual proliferation of the malaria parasite Plasmodium falciparum in the red blood cells (RBC) follows a developmental program that alternates intraerythrocytic replication within a parasitophorous vacuole (PV) with dissemination to new host cells. Egress from erythrocytes is timely stimulated by a cGMP- and calcium-dependent pathway that activates the cGMP-dependent protein kinase PfPKG and the calcium-dependent kinase PfCDPK5 respectively. This in turn triggers the exocytosis of secretory organelles, namely exonemes and micronemes, enabling the release of key effectors required for the rupture of the surrounding PV and host membranes. Preceding PfPKG activation, early PV morphological modifications have been described, including PV poration and PV swelling, also known as the “rounding-up” step, but the molecular effectors involved are still unknown.
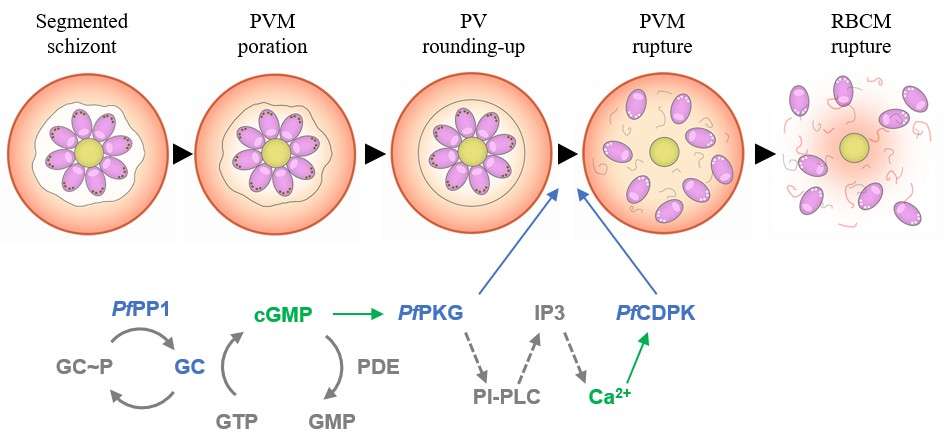
The egress scheme pathway in P. falciparum. Egress of the merozoites from the host red blood cell (RBC) requires the parasite to breach sequentially the surrounding membranes: the parasitophorous vacuole membrane (PVM) and the RBC membrane (RBCM). Following the end of schizogony (step 1), two morphological modifications of the PV take place, i.e. PVM poration and PV rounding-up (step 2). Then, an increase in cGMP concentration, likely regulated by the parasite phosphatase PP1, activates its downstream effector, the parasite kinase PKG that in turn leads to an increase in intracellular calcium concentration activating the kinase CDPK5 (step 3). These kinases are required to trigger the exocytosis of specific parasite organelles, namely the exonemes and micronemes, thereby releasing key effectors of egress. PP1, PKG and CDPK5 are necessary for PVM rupture (step 3). Finally, it has been observed that one single pore is formed and stabilized in the RBCM and that curling and buckling of this membrane allows the efficient release and dispersion of the merozoites in the extracellular milieu (steps 4 and 5). In addition to the pore formation, destabilization of the RBC cytoskeleton by parasite proteins is also required. Source from Collins et al. 2013, Brochet et al. 2014, Yeoh et al. 2007, Dvorin et al. 2010, Garg et al. 2013, Absalon et al. 2018, Abkarian et al. 2011, Das et al. 2015, Thomas et al. 2018, Hale et al. 2017, Glushakiva et al. 2018, Paul et al. 2020.
We have identified the protein phosphatase PP1 (PfPP1) as a master regulator of the egress signaling pathway6. A DiCre conditional mutant line for PfPP1 (PfPP1-iKO), fails to rupture the PV membrane (PVM) due to an incapacity to secrete exonemes and micronemes. A phosphoproteomic analysis of PfPP1 mutant revealed phosphoregulation of guanylate cyclase (GC), the enzyme responsible for cGMP synthesis, thereby placing PfPP1 upstream of the well characterized PfPKG in the egress signaling cascade.
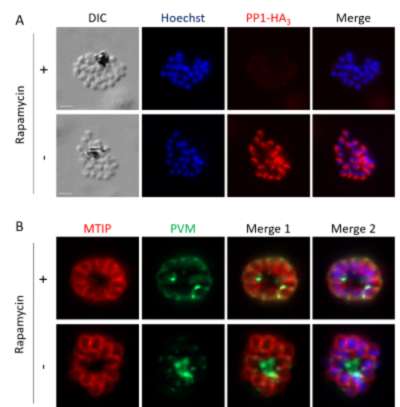
Figure: PP1 is required for PVM rupture at the time of egress.
(A) IFA showing the depletion of the protein PP1-HA3 upon rapamycin treatment in the inducible PP1 knock-out line (PP1-iKO), based on the conditional excision of pp1 gene by a dimerizable Cre recombinase. (B) IFA showing that in control parasites (- rapamycin), PVM rupture takes place as shown by the detection of membrane whorls, in contrast to PP1-depleted parasites (+ rapamycin) that remain enclosed within the PVM.
As the mechanistic of the early steps of egress is poorly understood, our project aims at using PP1 to expand our knowledge of the egress pathway and to explore its contribution in the biology of Plasmodium sexual stages, especially during gametocytes egress. For this, we will combine cutting-edge technologies in cellular and molecular parasitology to precisely define the egress step controlled by PP1, to evaluate PP1 contribution in sexual stages development, to identify egress-specific targets of the phosphatase and to determine to which extent those targets represent new molecular effectors of the egress signaling pathway. The better comprehension of PP1 functions in Plasmodium biology and egress may open the avenue for future intervention strategy.