Host cell invasion and rhoptry secretion
Head of group: Dr. Maryse Lebrun
Host Cell invasion
The Apicomplexa phylum is defined by the presence of an apical complex of organelles comprising two specific secretory granules called micronemes and rhoptries, which sequentially release their contents during invasion. Microneme proteins contribute to motility, attachment and invasion, while rhoptry proteins contribute to both invasion and subsequent manipulation of host cell functions after the parasites have established themselves in their host cells. Our research is focused on the formation of a structure (the moving junction) which is essential for host cell invasion in both Toxoplasma and Plasmodium, and which depends on the cooperation between microneme and rhoptry proteins. We are also interested on the mechanism of secretion of rhoptries, which remains a major conundrum in the field.
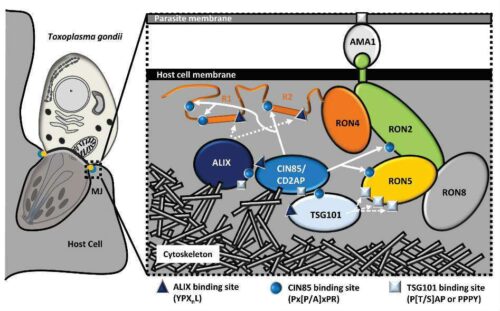
Schematic of T. gondii tachyzoite invading a host cell. Details in the inset show how, in the MJ, AMA1 is exposed on the parasite surface and interacts with a short extracellular domain of RON2 incorporated into the membrane of the host cell. The complex of RON2, RON4, RON5 and RON8 localize to the host cytosol and cooperate to recruit adaptor proteins (ALIX, CD2AP, CIN85, TSG101) through multiple specific interaction motifs that physically link the RONs complex to the cortical actin cytoskeleton. This results in the stabilization of the junction between the parasite and the host cortical actin providing a substantial cellular anchor for the AMA1/RON2 bridge at the surface, which is necessary to sustain the parasite invasive force.
Rhoptry secretion
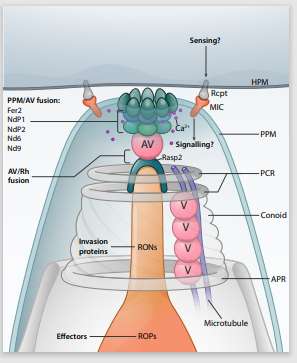
The ability of Apicomplexa to cause disease depends on the coordinated secretion of specialized secretory organelles. The rhoptries are particularly important, because they act as the apicomplexan equivalent of bacterial secretion systems. They inject parasite proteins directly in the cytoplasm of host cells not only for invasion but also to hijack host functions crucial to establish and maintain infection. However, in contrast to bacteria where the secretion machinery has been resolved in details at the atomic scale, how these eukaryotic parasites secrete and inject rhoptry effectors into cells is still an enigma. We aim to dissect the mechanistic steps and the molecular components that assemble the rhoptry secretion machine.
Funding
The group has been been awarded ERC Advanced grant 2018 over 5 years by the European Council to unravel the mechanism of eukaryote secretion system.
