Research projects – Team 6
GROUP 1: IMMUNE CELL ACTIVATION
Group leader : Dr. Mai NGUYEN CHI
Innate immunity is at the front line to thwart microorganism invasion. The zebrafish has proven particularly suitable for studying immune response to infections and injury. Thanks to the genetic amenability and transparency of its larvae and embryos, it provides an outstanding opportunity to decipher the dynamics of immune cell activation in infected and damaged tissues.
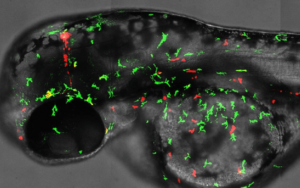
Our group uses the zebrafish larvae to unravel how phagocytes differentiate and fight microbes.
GROUP 2: DANGER SIGNALS AND CHRONIC INFLAMMATION
Group leader : Dr. Laure YATIME
Inflammation is a natural process generated by our immune system in response to an external or internal aggression. A healthy organism is usually able to down-tune it and restore homeostasis when the threat is eliminated. Conversely, uncontrolled inflammation can be deleterious to the body and cause serious damage, potentially leading to diseases or exacerbating an underlying pathology if the inflammation becomes chronic.
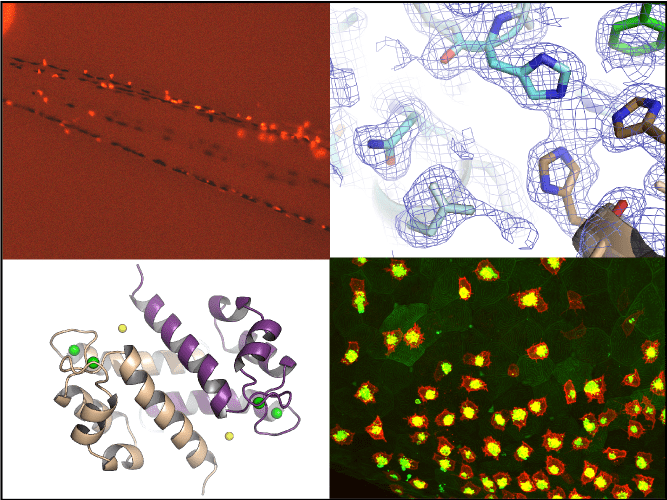
We are interested in the molecular actors of the innate immune system whose excessive activation by pathogenic or endogenous danger signals causes chronic inflammation related to human pathologies such as hemolytic diseases, inflammatory bowel diseases or cancers. Our current work aims to understand how the recognition of these danger signals by specific immune receptors generates a pro-inflammatory response promoting the progression of these pathologies. To this purpose, we use a multidisciplinary approach combining biochemistry, cell biology, structural biology and in vivo modeling in zebrafish.
We develop the zebrafish embryo model to define the involvement of this intramacrophage phase in the establishment and persistence of the infection, in a normal or CF context. We are also using this vertebrate model to test new strategies to limit P. aeruginosa infection.
GROUP 3: CELLULAR COMMUNICATION IN THE HEMATOPOIETIC NICHE
Group leader : Dr. Etienne LELIEVRE
Following their emergence from the aorta hemogenic endothelium, hematopoietic stem cells (HSCs) join and seed a transient hematopoietic organ, the so-called caudal hematopoietic tissue (CHT). The CHT is a complex vascular plexus made of endothelial cells, stromal cells and neuronal extensions that allows HSC expansion and differentiation to give rise to progenitors as well as to mature hematopoietic cells.
HSCs homing in the CHT involves physical interactions with both endothelial and stromal cells triggering the rearrangement of endothelial cells to form “stem cell pockets” that provide the correct environment for HSCs proliferation and a proper balance between stemness maintenance and differentiation.
Our work is currently focusing on the characterization of the mechanisms at work in fish deficient for components of TGF-beta/BMP superfamily that display compromised HSCs engraftment in the CHT. Our current results will provide grounds to a broader project aimed at understanding at both cellular and molecular levels how HSCs, endothelial cells, stromal cells and nerves dialog to establish a fully operative hematopoietic niche.